General Overview: Biology relies on quickly adapting to environmental and developmental cues. Large cells such as oocytes and neurons can rapidly alter their proteomes by storing mRNAs in RNA granules distributed throughout the cytoplasm and activating translation when needed. A number of reproductive and neuronal disorders are caused by misregulated translation. By understanding how disease factors control translation at the molecular level, we aim to open a path towards therapeutics for autism and ovarian disorders.
We have developed a model system for studying translational control in its "purest" state in vivo using mature Drosophila oocytes. Mature oocytes are giant cells which are transcriptionally inactive and rely entirely on the translation of stored mRNAs. We study how oocytes control the translation of their stored mRNAs by (1) characterizing translation genome-wide using ribosome profiling and RNA sequencing in wild type and mutant backgrounds (2) identifying novel factors using genetic screens and cell-type specific perturbations, and (3) discovering roles for translation factors in development and aging by studying effects on oogenesis and early embryonic development.
We have developed a model system for studying translational control in its "purest" state in vivo using mature Drosophila oocytes. Mature oocytes are giant cells which are transcriptionally inactive and rely entirely on the translation of stored mRNAs. We study how oocytes control the translation of their stored mRNAs by (1) characterizing translation genome-wide using ribosome profiling and RNA sequencing in wild type and mutant backgrounds (2) identifying novel factors using genetic screens and cell-type specific perturbations, and (3) discovering roles for translation factors in development and aging by studying effects on oogenesis and early embryonic development.
Key insights made thus far include:
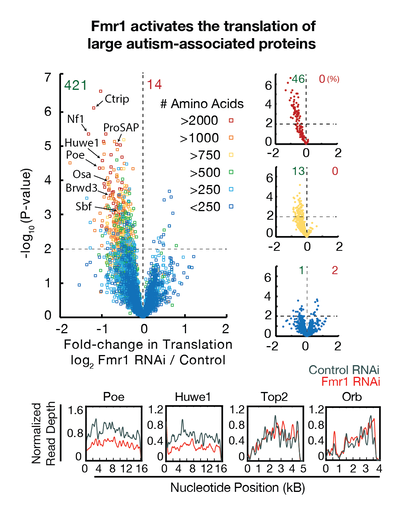
– We found that Fmr1, a gene mutated in the most common autism and ovarian disorders, is part of a system that translates many of the largest proteins encoded in the genome (Greenblatt and Spradling 2018). We are interested in understanding how this system works, and whether restoring the normal balance between translational activators such as Fmr1 and translational repressors can ameliorate molecular defects underlying ovarian and autism spectrum disorders.
– We found that Fmr1, a gene mutated in the most common autism and ovarian disorders, is part of a system that translates many of the largest proteins encoded in the genome (Greenblatt and Spradling 2018). We are interested in understanding how this system works, and whether restoring the normal balance between translational activators such as Fmr1 and translational repressors can ameliorate molecular defects underlying ovarian and autism spectrum disorders.
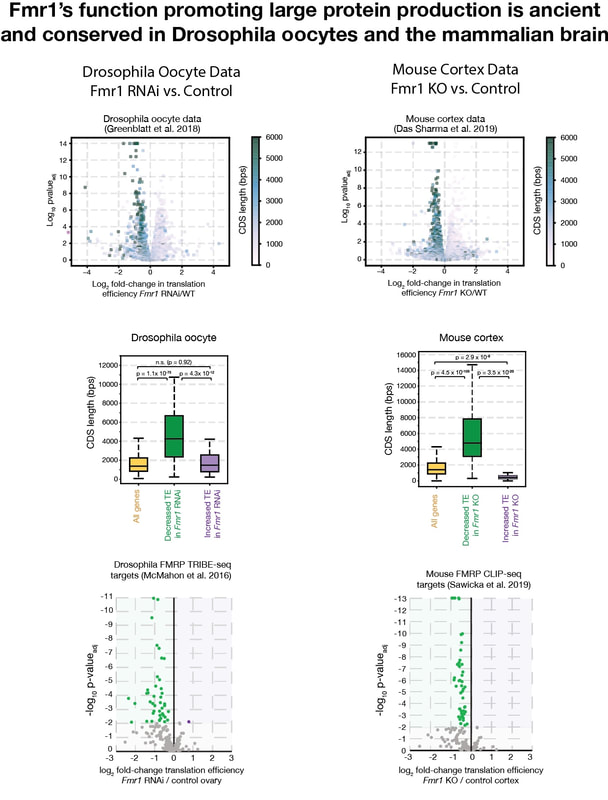
- We have found that FMRP's function boosting the production of large proteins is conserved from fly eggs to the mammalian brain (Flanagan et al., 2022a in press). Our comparisons of mouse cortex data (Das Sharma et al. 2019) with our fly studies show that large proteins are selectively reduced in translation to a similar magnitude in the absence of FMRP in both tissues. In collaboration with Khan Dao Duc's lab at UBC, we developed new methods for determining from ribosome profiling data whether translation factors act at the level of initiation or elongation (Flanagan et al. 2022b, in revision). These studies confirm that FMRP functions specifically as a translation initiation factor.
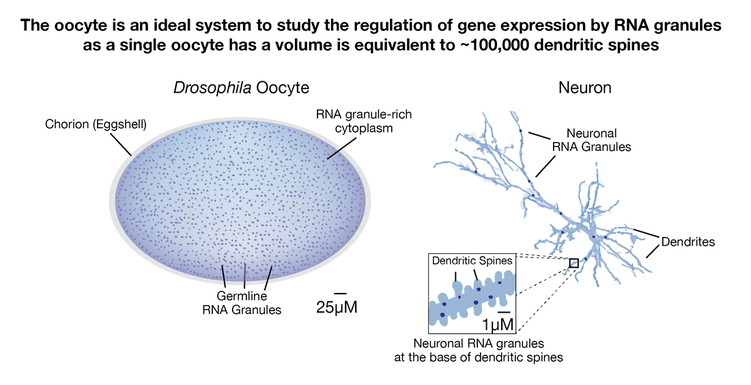
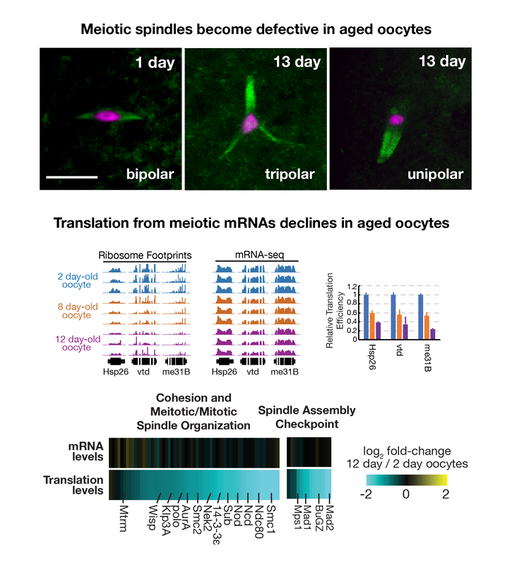
– Oocytes contain large pools of mRNAs, which are preserved for several weeks. While mRNA levels are stable, translation declines substantially during aging (>50%), and this reduction is associated with meiotic failure and infertility (Greenblatt et al. 2019). During development human oocytes also cease transcription (~10 weeks prior to maturation). We hypothesize that the inability to maintain sufficient translation from long-lived mRNAs leads to age-associated meiotic errors and follicle loss that limits human reproductive lifespan.
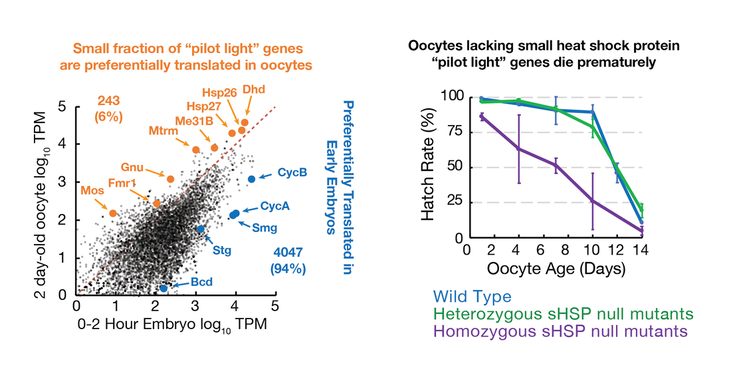
– While the vast majority of genes are translationally repressed in oocytes, 6% of genes are translationally upregulated specifically during the oocyte's prolonged developmental arrest (Greenblatt et al. 2019). Some of these genes - such as Fmr1, Hsp26 and Hsp27 act as "pilot lights," prolonging oocyte function during extended periods of developmental arrest.
Current projects:
(1) What is the mechanism of Fmr1-dependent translational activation? What are the role(s) of Fmr1 granules and stress granules/P bodies in the translational control of large proteins? How does Fmr1 select its targets and can we find genetic suppressors that rescue Fmr1's mutant phenotypes?
(2) How are mRNA molecules maintained for weeks? Are there RNA chaperones which sustain mRNA translation? Why does translation decline during aging and what are the roles for translational decline in reproductive failure and neurodegeneration? Do mRNA molecules naturally aggregate during aging in a manner akin to protein aggregation?
(3) What are the roles of "pilot light" genes in reproductive maintenance? What are the critical processes that are maintained during cellular quiescence and does the inability to maintain these processes contribute to reproductive failure?
(1) What is the mechanism of Fmr1-dependent translational activation? What are the role(s) of Fmr1 granules and stress granules/P bodies in the translational control of large proteins? How does Fmr1 select its targets and can we find genetic suppressors that rescue Fmr1's mutant phenotypes?
(2) How are mRNA molecules maintained for weeks? Are there RNA chaperones which sustain mRNA translation? Why does translation decline during aging and what are the roles for translational decline in reproductive failure and neurodegeneration? Do mRNA molecules naturally aggregate during aging in a manner akin to protein aggregation?
(3) What are the roles of "pilot light" genes in reproductive maintenance? What are the critical processes that are maintained during cellular quiescence and does the inability to maintain these processes contribute to reproductive failure?
Watch us discussing our work on fragile X syndrome: